Autophagy, Mitochondria and Extracellular Vesicles
Autophagy is a bulk degradation system involving lysosomes that was discovered in the late 1950s. The term “autophagy” was coined as a self-digestion mechanism by Professor Duve in 1963. From the 1950s to the 1980s, many researchers observed autophagosomes delivering intracellular components to the lysosome; however, the mechanism remained obscure. In 1993, the stagnation was broken by Professor Ohsumi, a Nobel laureate. Using a genetic screen for yeast mutants, Professor Ohsumi discovered 14 genes that are essential for autophagy. In the years since, researchers have intensively investigated this field, identifying many autophagy-related genes in mammals and clarifying their physiological and pathological roles. Autophagy is considered a major quality-control mechanism essential for homeostasis, development, and protection against a variety of stressors.
Among the various autophagic pathways, mitophagy (mitochondrial autophagy) has been the most studied because mitochondrial quality control is vital for cellular viability. The Parkin-Pink1 dependent mechanism has attracted attention in the past; however, accumulating evidence suggests that mitophagy is executed through multiple pathways in a context-dependent manner, and Parkin-Pink1 is not indispensable under some conditions.
PI is one of the most recognized researchers in this field, in light of the discovery of Ulk1-Rab9-dependent mitophagy in the heart under conditions of ischemia or metabolic stress (Figure).
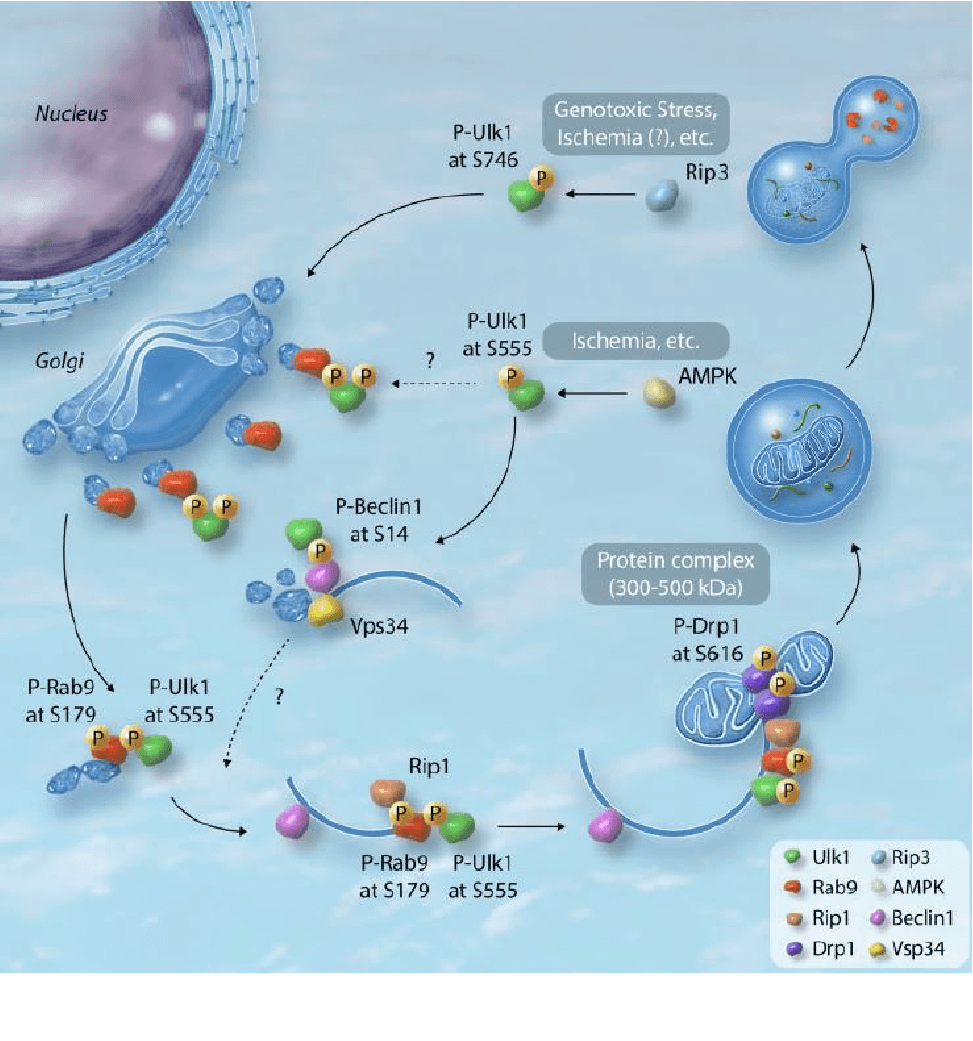
Recent studies have revealed that extracellular vesicles (EVs), which are major actors in intra-organ communication, are capable of delivering mitochondria. Using these EVs, adipose tissues send their mitochondria to target organs, including the heart, to help regulate the redox state in remote organs.
We were very excited to learn of this report, as we have a strong interest in this field and possess novel reporter mice tracking EVs. We are now elucidating the characteristics of EVs originating from various organs and clarifying whether and how they affect the pathophysiology of some stressors, such as hypertension, metabolic disorders, and senescence.